How To Draw A Titration Curve
| pH (TITRATION) CURVES This folio describes how pH changes during various acid-base titrations. The equivalence point of a titration Sorting out some disruptive terms When you carry out a uncomplicated acrid-base titration, y'all use an indicator to tell you when you take the acid and alkali mixed in exactly the right proportions to "neutralise" each other. When the indicator changes color, this is ofttimes described as the finish point of the titration. In an ideal world, the colour change would happen when you mix the two solutions together in the correct proportions co-ordinate to the equation (often just called "equation proportions"). That particular mixture is known equally the equivalence point . For example, if y'all were titrating sodium hydroxide solution with hydrochloric acid, both with a concentration of 1 mol dm-3, 25 cmthree of sodium hydroxide solution would need exactly the same volume of the acrid - considering they react 1 : 1 according to the equation. In this item instance, this would as well be the neutral bespeak of the titration, because sodium chloride solution has a pH of 7. But that isn't necessarily true of all the salts yous might get formed. For example, if you titrate ammonia solution with hydrochloric acrid, you would get ammonium chloride formed. The ammonium ion is slightly acidic, and and then pure ammonium chloride has a slightly acidic pH. That means that at the equivalence point (where you had mixed the solutions in the correct proportions co-ordinate to the equation), the solution wouldn't really exist neutral. To use the term "neutral point" in this context would exist misleading. Similarly, if you titrate sodium hydroxide solution with ethanoic acrid, at the equivalence point the pure sodium ethanoate formed has a slightly alkali metal pH because the ethanoate ion is slightly basic. To summarise:
| |
| Note:You can find out about indicators by following this link (also available from the acid-base of operations equilibria card). You should read the present page first though. | |
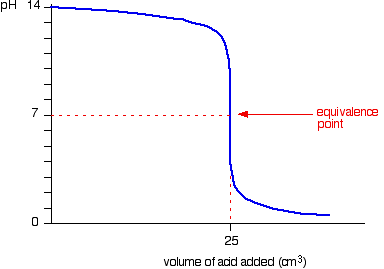
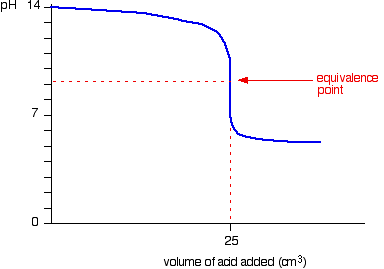
| Elementary pH curves All the following titration curves are based on both acid and alkali having a concentration of 1 mol dm-3. In each case, you start with 25 cmiii of 1 of the solutions in the flask, and the other one in a burette. Although you usually run the acrid from a burette into the alkali in a flask, you may demand to know almost the titration curve for adding it the other way effectually as well. Alternative versions of the curves accept been described in most cases. Titration curves for potent acid five potent base We'll have muriatic acid and sodium hydroxide equally typical of a strong acid and a strong base of operations. Running acid into the alkali Y'all can run across that the pH merely falls a very small corporeality until quite virtually the equivalence point. Then there is a really steep plunge. If you calculate the values, the pH falls all the mode from 11.3 when yous accept added 24.ix cm3 to ii.7 when you take added 25.1 cm3. | |
| Note:If you need to know how to calculate pH changes during a titration, yous may be interested in my chemistry calculations book. | |
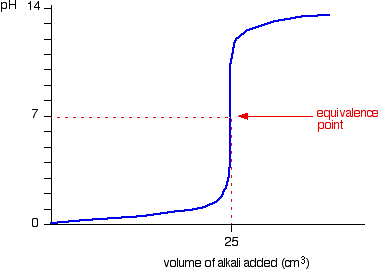
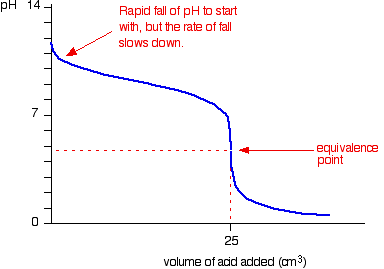
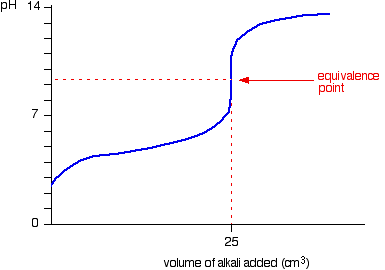
| Running alkali into the acid This is very similar to the previous bend except, of course, that the pH starts off low and increases as you add more sodium hydroxide solution. Once more, the pH doesn't change very much until you lot get shut to the equivalence point. And so it surges upwards very steeply. Titration curves for strong acid v weak base This time we are going to use hydrochloric acid every bit the stiff acrid and ammonia solution as the weak base. Running acrid into the alkali Because you take got a weak base, the beginning of the curve is obviously going to be dissimilar. Withal, in one case you lot accept got an backlog of acid, the curve is essentially the same every bit before. At the very beginning of the curve, the pH starts by falling quite chop-chop as the acid is added, just the curve very soon gets less steep. This is because a buffer solution is being set up - equanimous of the excess ammonia and the ammonium chloride being formed. | |
| Annotation:Yous can find out more than about buffer solutions by post-obit this link. However, this is a very minor point in the present context, and you would probably exercise better to read the whole of the electric current page before you follow this upwards. | |
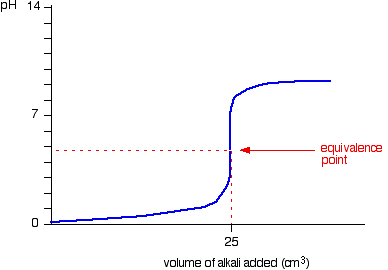
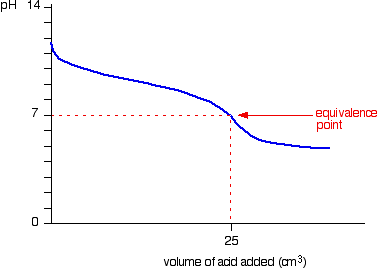
| Notice that the equivalence betoken is now somewhat acidic ( a bit less than pH v), because pure ammonium chloride isn't neutral. Withal, the equivalence point all the same falls on the steepest bit of the curve. That will turn out to be important in choosing a suitable indicator for the titration. Running alkali into the acid At the beginning of this titration, you accept an backlog of muriatic acid. The shape of the curve will be the aforementioned as when you had an excess of acid at the start of a titration running sodium hydroxide solution into the acid. Information technology is just after the equivalence point that things become different. A buffer solution is formed containing excess ammonia and ammonium chloride. This resists any big increase in pH - not that you would expect a very big increase anyway, considering ammonia is only a weak base. Titration curves for weak acid v strong base We'll take ethanoic acid and sodium hydroxide as typical of a weak acrid and a strong base. Running acrid into the alkali For the first part of the graph, y'all have an excess of sodium hydroxide. The bend will be exactly the same as when you add muriatic acid to sodium hydroxide. In one case the acid is in excess, in that location volition be a difference. By the equivalence point you accept a buffer solution containing sodium ethanoate and ethanoic acrid. This resists whatsoever big fall in pH. Running alkali into the acid The first of the graph shows a relatively rapid ascent in pH but this slows down as a buffer solution containing ethanoic acrid and sodium ethanoate is produced. Beyond the equivalence bespeak (when the sodium hydroxide is in excess) the curve is just the same equally that end of the HCl - NaOH graph. Titration curves for weak acid v weak base The common example of this would be ethanoic acid and ammonia. It so happens that these two are both nearly equally weak - in that case, the equivalence point is approximately pH seven. Running acid into the alkali This is really merely a combination of graphs you have already seen. Upwardly to the equivalence point it is similar to the ammonia - HCl instance. After the equivalence point information technology is like the finish of the ethanoic acrid - NaOH curve. Detect that there isn't any steep flake on this graph. Instead, there is only what is known as a "point of inflexion". That lack of a steep chip ways that information technology is difficult to practise a titration of a weak acid against a weak base of operations. | |
| Note:Because y'all about never do titrations with this combination, there is no real point in giving the graph where they are added the other way round. It isn't difficult to work out what it might await like if you lot are interested - take the commencement of the sodium hydroxide added to ethanoic acid curve, and the end of the ammonia added to hydrochloric acid one. The reason that information technology is difficult to do these titrations is discussed on the page about indicators. | |
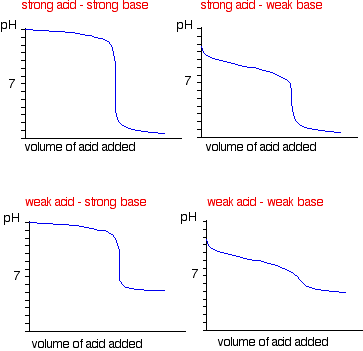
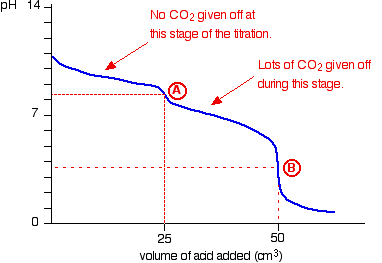
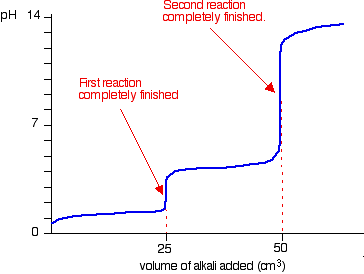
| A summary of the important curves The fashion you normally carry out a titration involves adding the acid to the alkali. Hither are reduced versions of the graphs described in a higher place and so that y'all can run into them all together. More complicated titration curves Calculation hydrochloric acid to sodium carbonate solution The overall equation for the reaction betwixt sodium carbonate solution and dilute hydrochloric acid is: If you had the two solutions of the same concentration, you lot would have to employ twice the volume of hydrochloric acid to reach the equivalence point - because of the ane : ii ratio in the equation. Suppose you start with 25 cm3 of sodium carbonate solution, and that both solutions have the same concentration of 1 mol dm-3. That means that you would await the steep drib in the titration curve to come after y'all had added 50 cmiii of acid. The actual graph looks similar this: The graph is more complicated than you might think - and curious things happen during the titration. You await carbonates to produce carbon dioxide when you lot add acids to them, but in the early stages of this titration, no carbon dioxide is given off at all. So - as before long every bit you get past the half-way point in the titration - lots of carbon dioxide is suddenly released. The graph is showing two end points - one at a pH of eight.three (little more than a signal of inflexion), and a second at about pH iii.7. The reaction is plain happening in two distinct parts. In the first function, complete at A in the diagram, the sodium carbonate is reacting with the acrid to produce sodium hydrogencarbonate: You can encounter that the reaction doesn't produce whatever carbon dioxide. In the 2d role, the sodium hydrogencarbonate produced goes on to react with more acid - giving off lots of COtwo. That reaction is finished at B on the graph. It is possible to selection upwardly both of these end points by conscientious pick of indicator. That is explained on the separate page on indicators. Calculation sodium hydroxide solution to dilute ethanedioic acrid Ethanedioic acrid was previously known as oxalic acid. It is a diprotic acid , which means that it can requite away 2 protons (hydrogen ions) to a base of operations. Something which can only give abroad one (like HCl) is known as a monoprotic acid. The reaction with sodium hydroxide takes place in 2 stages considering one of the hydrogens is easier to remove than the other. The two successive reactions are: If y'all run sodium hydroxide solution into ethanedioic acid solution, the pH curve shows the end points for both of these reactions. The curve is for the reaction between sodium hydroxide and ethanedioic acrid solutions of equal concentrations. Questions to test your understanding If this is the start set of questions you have done, please read the introductory page before you lot starting time. You will demand to utilise the Dorsum Push on your browser to come back here afterward. questions on pH curves answers
To the acid-base equilibria carte . . . To the Concrete Chemistry carte . . . To Primary Menu . . . © Jim Clark 2002 (modified November 2022) | |
Source: https://www.chemguide.co.uk/physical/acidbaseeqia/phcurves.html
Posted by: maravillamilt1943.blogspot.com

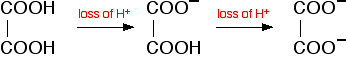

0 Response to "How To Draw A Titration Curve"
Post a Comment